Science in the field - how do we measure greenhouse gasses? [methods]
- nicolakokkonen

- Aug 13, 2025
- 3 min read
Gasses can be hard to see, feel, or otherwise catch, especially in really really small amounts, so how do we do it?

Methane and carbon dioxide - a background
These two gasses are arguably the most important gasses in our atmosphere in regards to climate change. Carbon dioxide (CO2) is the most prevalent greenhouse gas and is usually linked to human activities, such as burning fuels. In nature, carbon dioxide is emitted when fungi, bacteria, and other microbes break down organic material (such and plant material) in an environment with oxygen. It also is released by plants as they use sugars to grow. Naturally, carbon dioxide is consumed by plants during photosynthesis.
Methane (CH4), is much less common than carbon dioxide, but it is a much more powerful at causing atmospheric warming. It is also a product of human activities (farming, burning fuels), but methane can also be produced in nature when organic material is broken down in an environment without oxygen, such as a wet peatland or lake bottom. Natural bacteria and microbes in the soil (called methanotrophs) will remove methane from the atmosphere by consuming it (oxidation).

How to measure?
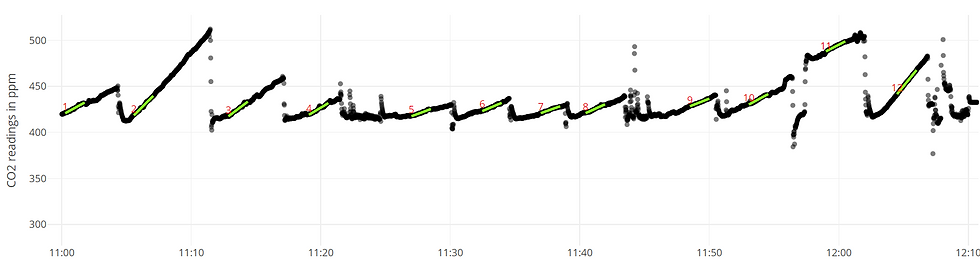
Until recently, scientists who measured greenhouse gasses had to capture samples of the gas, take them back to a laboratory, and analyze each sample using a gas chromatograph. A gas chromatograph is able to measure the concentration of many different gasses in an air sample using the unique physical and chemical properties of gasses such as boiling point and polarity. Samples from different environments can be compared, as can samples from different points in time. Comparing gas concentrations in a closed environment (like a sealed bottle) over time can be used to calculate how fast gasses are being produced or consumed in a closed environment. This is the foundation of modern measurements too, although different methods are now available.
Nowadays, portable trace gas analyzers that use lasers to give instantaneous gas readouts are very popular due to their speed. ease of use, and suitability for lab and field conditions. These lasers rely on the light absorption properties of different gasses to measure their concentration in the air that flows through the analyzer from a closed environment. Now, instead of collecting samples and sending them to a lab, researchers are able to immediately see how specific gasses are being released or absorbed in an environment.

What does this mean in practice?
I use a portable trace gas analyzer in my work to measure carbon dioxide and methane concentrations in the air in a sealed chamber on the ground in the forest. This means that I have collars (plastic or metal rings) in the ground that work like a sealed foundation. On these collars I place a chamber that is secured with a metal ring to make a closed system, i.e. no air leaks in or out and we know how big it is. The gas analyzer is connected to this chamber with two hoses; one that takes air out of the chamber and in to the analyzer, and the other that takes air out of that analyzer and back into the chamber. This way, air is not entering or leaving and if the ground is producing a gas, the concentration will continue to grow and grow over time. Conversely, if the ground is absorbing a gas, the concentration in the chamber and analyzer will become less and less over time. This is how we know how much gas is released or absorbed by the soil (in a given space and time).
To do a measurement, I place the chamber on the collar, and start recording the gas concentration. I wait for a couple of minutes and measure some other soil properties at the site, such as temperature and moisture, then I stop the measurement and remove the chamber. Easy, the analyzer takes care of the rest!




Comments